Hangzhou,Zhejiang,China China
 Water Soluble Coenzyme Q10 10% coq10 powder
Water Soluble Coenzyme Q10 10% coq10 powder
 12 Cups Fireless Cupping Therapy
12 Cups Fireless Cupping Therapy
 2012 royal smoke electronic cigarettes 803 PCC
2012 royal smoke electronic cigarettes 803 PCC
 24 Cups Fireless Cupping Therapy
24 Cups Fireless Cupping Therapy
 99.9% Nicotine
99.9% Nicotine
 Absorbent Cotton Roll
Absorbent Cotton Roll
 Acu pen (MG200)
Acu pen (MG200)
 Adjustable neck brace support for Injury Pain
Adjustable neck brace support for Injury Pain
 Agar agar
Agar agar
 Airial Digital Blood Pressure Monitor
Airial Digital Blood Pressure Monitor
Hangzhou Singclean Medical Products Co., Ltd. was
established in 2002, and currently has 18 years of expertise in the
industry of absorbable biomaterial with nearly 400 employees. The
production base covers an area of 28,000 square meters, with 8 GMP
purification workshops of 2,000 square meters, a logistics center
of 4,000 square meters, and a R&D center and a quality
inspection center of 1,000 square meters. Singclean has an annual
production capacity of 12 million sets of various medical devices,
and can achieve an output value of 300 million euros with an annual
sales over 61.8 million euros.
Singclean Medical has always put quality at the first place.
High-precision analytical instruments like GC, GCMS, TA rheometer,
Malvern particle size analyzer are used by our skilled staff to
carry out tests for incoming and outgoing quality control.
Meanwhile, Singclean has a strong research and development team
with more than 40 experienced engineers. Each year, over 2.8
million dollars is invested into R&D to create pioneering
medical products, like tissue engineering products.
There are currently 8 series of products (medical sodium
hyaluronate gel products, chitosan products, cross-linked sodium
hyaluronate gel products, microporous polysaccharide products,
oxidized regenerated cellulose products, in vitro diagnostic
reagent products (immune method and biochemical method), collagen
sponge, regenerative medicine engineering basic material products)
with 50 varieties which are exported to more than 70 countries
around the world, such as: Italy, Russia, Turkey, Brazil,
Chile.
As of August 2020, Singclean has obtained 12 CE certificates, 15
medical device registration certificates and 33 patent
certificates. After 18 years of development and growth, Hangzhou
Singclean Medical Products Co., Ltd. has become the top three
manufacturers in China's hyaluronic acid industry, and is
determined to become a leader in the field of absorbable
biomaterials in China!
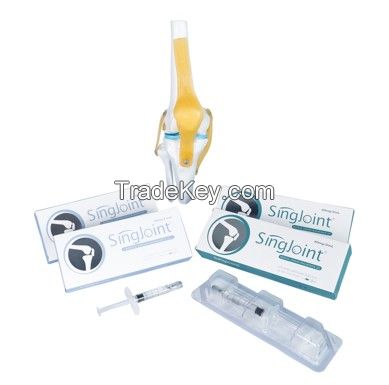 Singjoint
Singjoint
 Quickclean
Quickclean
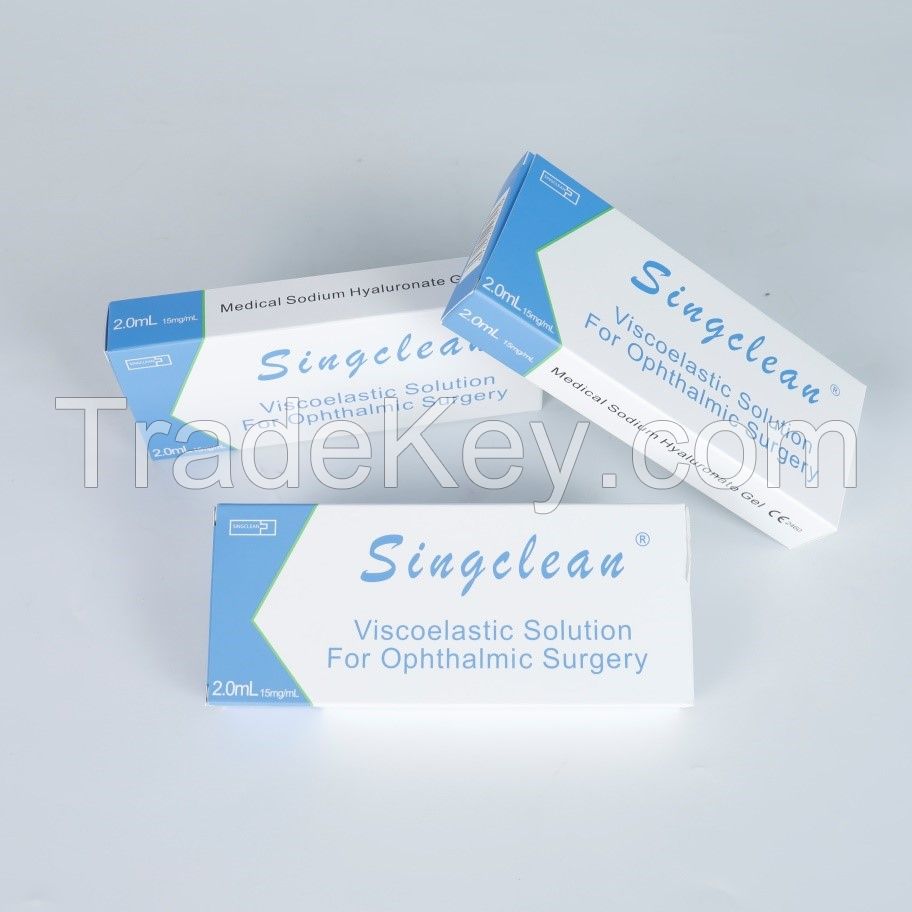 Singclean for ophthalmic surgery
Singclean for ophthalmic surgery
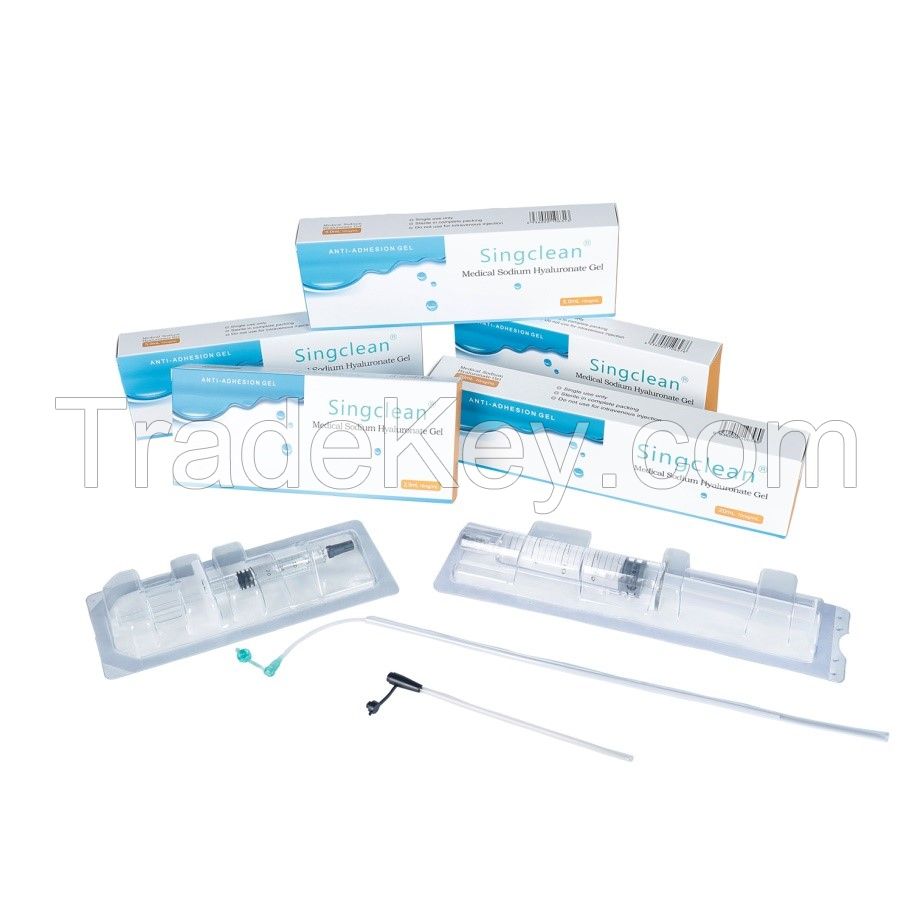 Singclean for anti-adhesion
Singclean for anti-adhesion
 Singfiller
Singfiller
 Singderm Dermal Filler
Singderm Dermal Filler
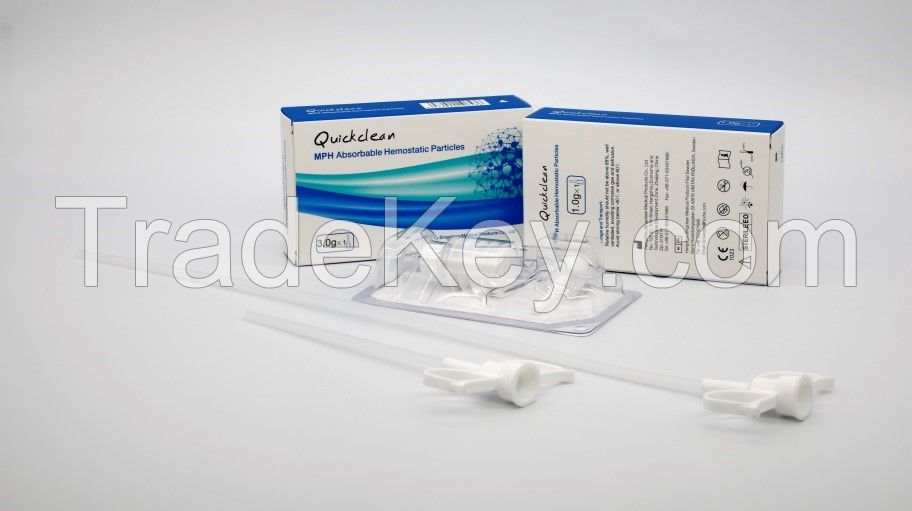 Quickclean MPH Absorbable Hemostatic Particles
Quickclean MPH Absorbable Hemostatic Particles
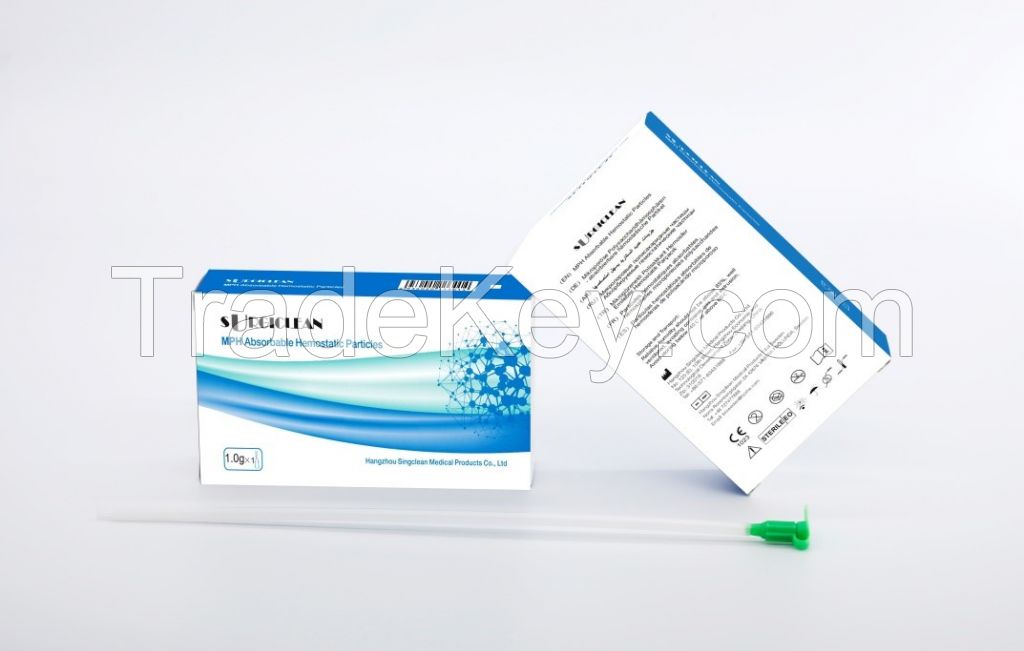 Surgiclean
Surgiclean
 Quickclean innovative applicators
Quickclean innovative applicators
 Surgiclean Absorptive Gauze of Oxidized Regenerated Cellouse
Surgiclean Absorptive Gauze of Oxidized Regenerated Cellouse
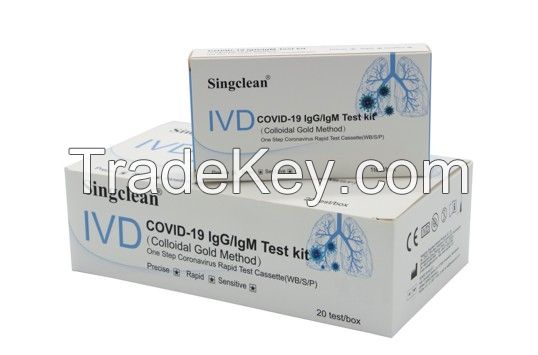 Singclean COVID-19 Igg/IgM Test Kit (Colloidal Gold Method)
Singclean COVID-19 Igg/IgM Test Kit (Colloidal Gold Method)
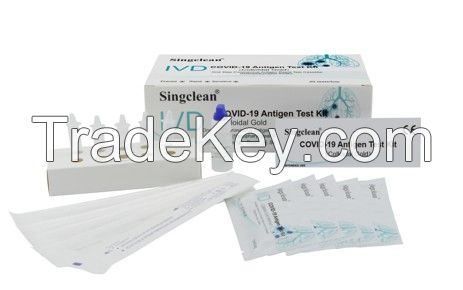 Singclean COVID-19 Test Kit Nasopharyngeal Swab
Singclean COVID-19 Test Kit Nasopharyngeal Swab
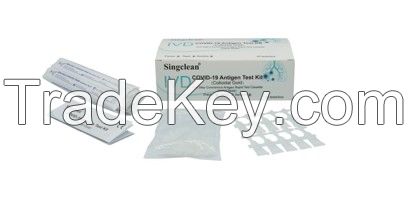 Singclean COVID-19 Antigen test kit Saliva Swab
Singclean COVID-19 Antigen test kit Saliva Swab
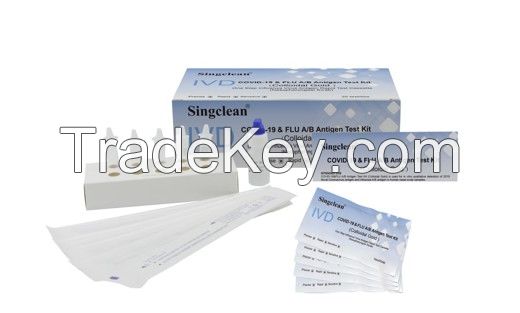 Singclean COVID-19 & Flue A/B Antigen Test Kit
Singclean COVID-19 & Flue A/B Antigen Test Kit
 Singclean Drug of Abuse (DOA) Test
Singclean Drug of Abuse (DOA) Test
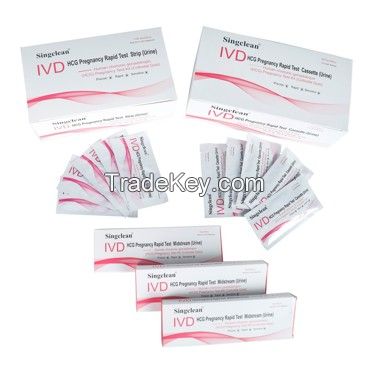 Singclean HCG Pregnancy Test Kit
Singclean HCG Pregnancy Test Kit
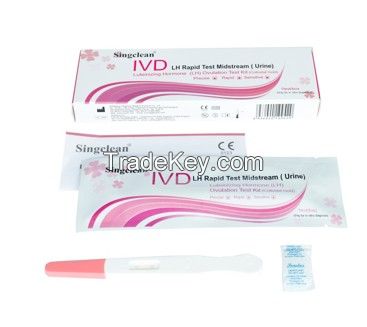 Singclean LH Ovulation Test
Singclean LH Ovulation Test
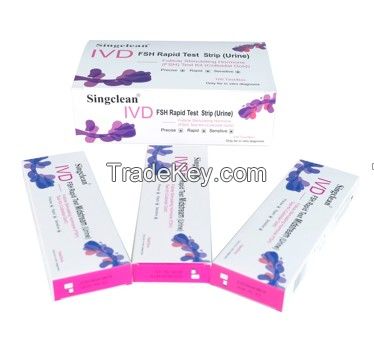 Singclean FSH test
Singclean FSH test
 Singclean Bacterial Vaginosis Test
Singclean Bacterial Vaginosis Test
 Singclean (AFP) Alpha-Fetoprotein ELISA Test
Singclean (AFP) Alpha-Fetoprotein ELISA Test
 Singclean Neutrophil Gelatinase-Associated Lipocalin Rapid Test Kit(Colloidal Gold)
Singclean Neutrophil Gelatinase-Associated Lipocalin Rapid Test Kit(Colloidal Gold)
 Singclean Microalbuminuria (MAU) Rapid Test Kit (Colloidal Gold)
Singclean Microalbuminuria (MAU) Rapid Test Kit (Colloidal Gold)
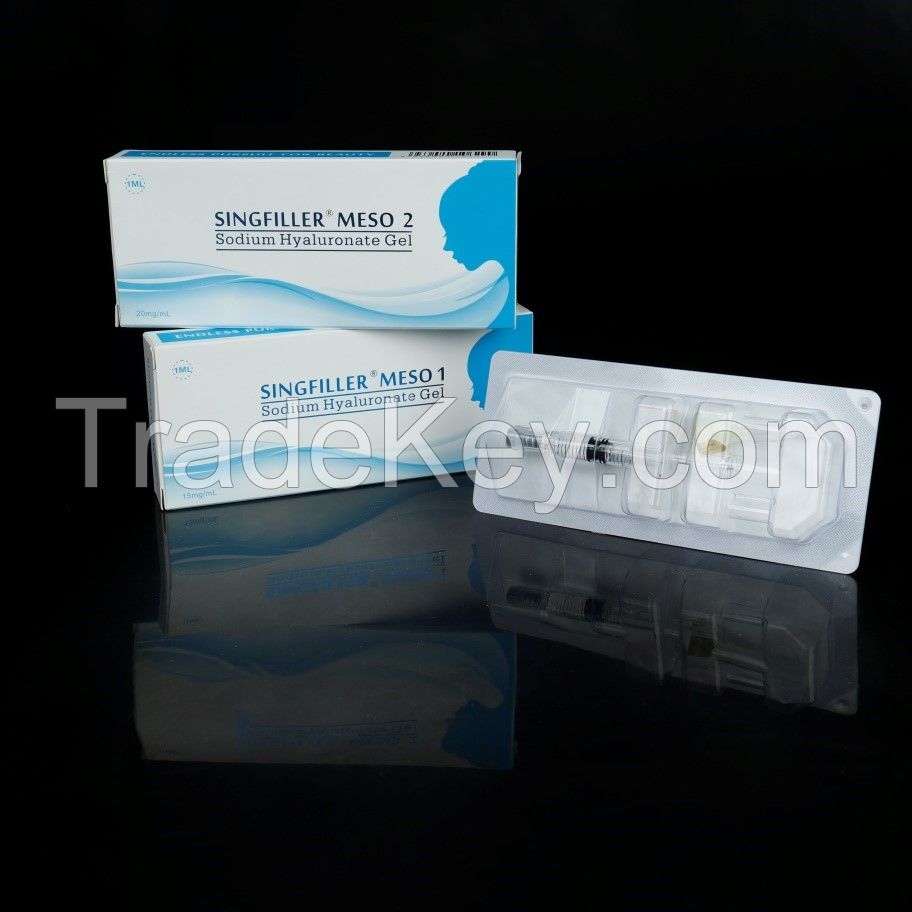 Singfiller MESO
Singfiller MESO
 Hyaclean Hyaluronic Acid Extract
Hyaclean Hyaluronic Acid Extract
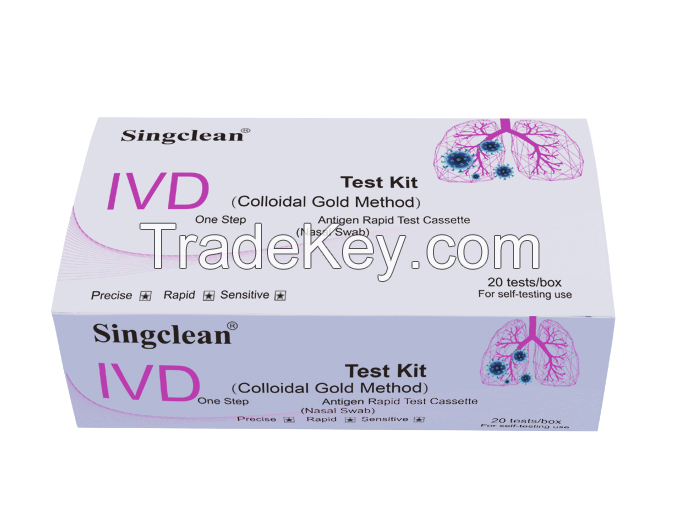 COVID-19 Test Kit Nasal Swab for self-testing use
COVID-19 Test Kit Nasal Swab for self-testing use
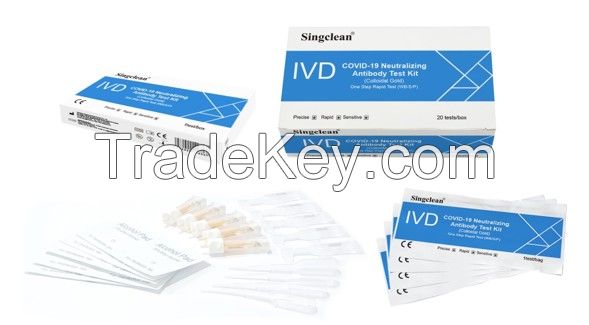 Singclean COVID-19 Neutralizing Antibody Test Kit
Singclean COVID-19 Neutralizing Antibody Test Kit
 Get Cheek Filler To Look Younger
Get Cheek Filler To Look Younger
 Give The Lips You Want To Kiss With Singderm Silk Lip Filler
Give The Lips You Want To Kiss With Singderm Silk Lip Filler
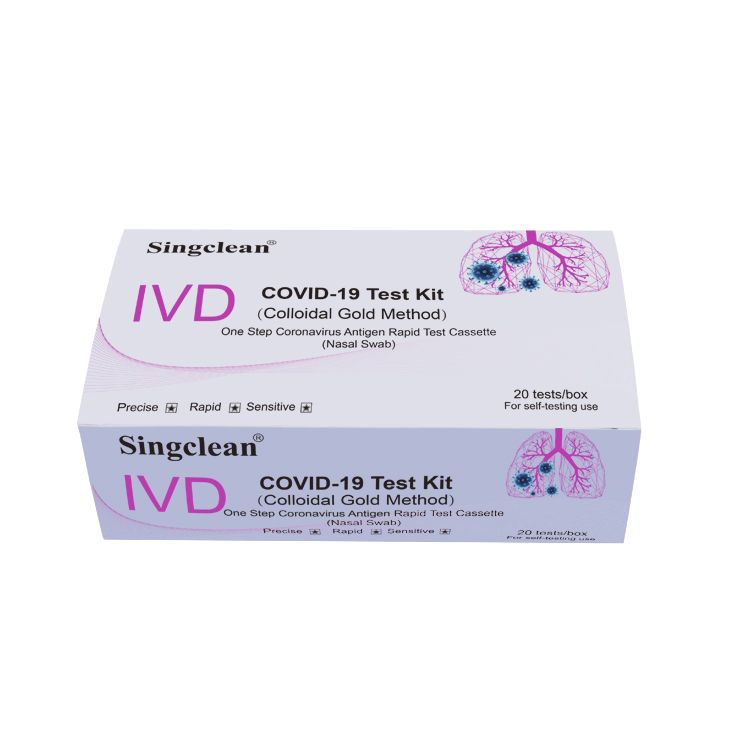 Singclean covid-19 test kit (colloidal gold method)��� for self-testing use
Singclean covid-19 test kit (colloidal gold method)��� for self-testing use
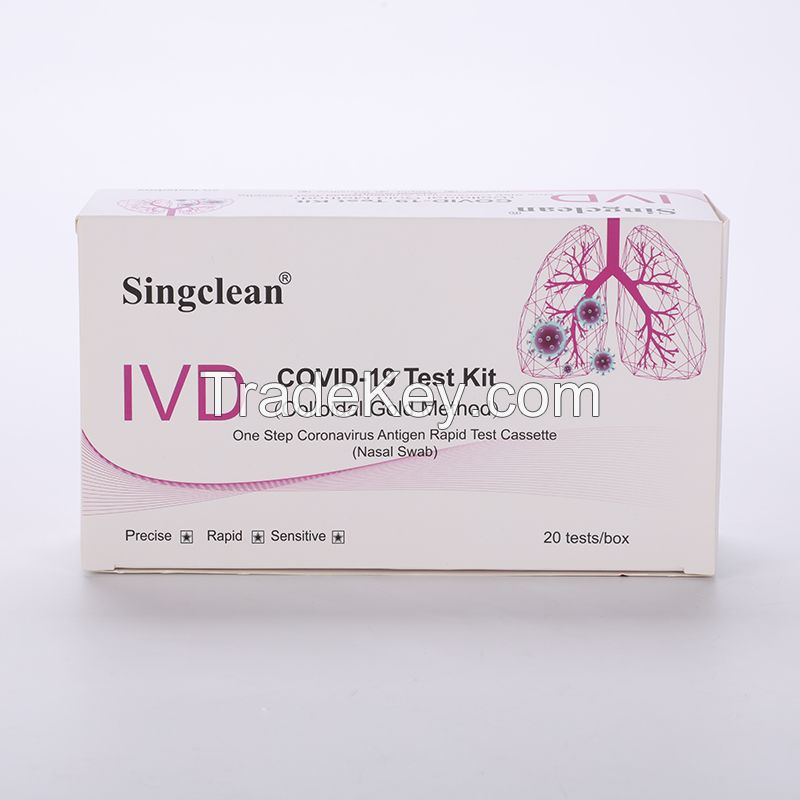 Covid-19 antigen test nasal swab with CE certificate for self testing
Covid-19 antigen test nasal swab with CE certificate for self testing
| 企业类型 | Manufacturing |
| 网站 | http://www.singclean.net/ |
| 建立年份 | 2002 |
| 公司雇员数量 | 101-500 |
| 主要市场 | Americas,Africa,Asia,Caribbean,America,Europe,Middle East,Oceania,Worldwide |
| 产品/服务 | hyalurnic acid,covid-19 test kit,absorbable hemostatic gauze,absorbable hemostatic particles,dermal filler,hyaluronic acid skin care products |
| 工厂位置 | Hangzhou,Zhejiang, China |
| 厂房面积 | 10000 sqm to 250000 sqm |
| 生产线号 | 7 |
| 年总购买量 | N/A |
| R&D人员数 | 31 - 40 people |
| 质量控制 | In House |
| 证明 | ISO13485,ISO9001,CE |
| 合同制造 | OEM Service Offered ,Buyer Label Offered |
| 注册资本 | 10 - 25 Million USD |
| 所有权类型 | Professional Association |
| 法人代表/执行总裁 | Abby Xu |
| 出口比例 | 30% |
| 年营业额 | 50 - 100 Million USD |
| 质检人数 | N/A |
| 联系人 | Ms. Bette |
| 公司名称 | Hangzhou Singclean Medical Products Co., Ltd. |
| 电话号码 | ******** |
| 移动电话 | ******** |
| 传真号码 | ******** |
免责声明:本视频由该供应商提供。 TradeKey.com对会员发布的任何内容不承担任何责任。